|
| |
AimStick™ PBD/AimStep™
hCG
Professional One-Step Early Detection
Pregnancy Tests
Specifications
and Testing Procedures
SPECIMEN
COLLECTION AND PREPARATION
The urine specimen must be collected in a clean, dry container, Specimens collected randomly may be used. However, the first morning urine generally contains the highest concentration of hormone. Urine specimens may be refrigerated (2° - 8°C) and stored up to 72 hours prior to assay. If specimens are refrigerated, they must be equilibrated to room temperature before testing. Urine samples exhibiting visible precipitates should be filtered, centrifuged, or allowed to settle (obtaining clear aliquots) before testing.
TEST PROCEDURE
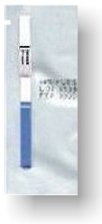
Materials
Provided:
|
|
Test
strip or cassette containing monoclonal mouse-hCG colored conjugate and goat
anti-hCG antibody pre-coated on a
porous test membrane. A suitable clean urine collection cup or device
(preferably glass)
for the test procedure should be provided by the test subject. AimStick™ One-step test strip
pre-packaged in foil pouch stamped with
lot# and expiration date. AimStep™
test cassettes are prepackaged with
a transfer pipette in fog
pouch stamped with lot# and expiration date. Both tests
are pre-calibrated at 20mIU/ml hCG sensitivity detection level. Both tests are
FDA approved and CLIA waived for professional and OTC use.
|

|
DIRECTIONS
FOR USE
-
Allow specimen and
test strip or cassette to reach room temperature (20° to 30°C) prior to
testing.
-
Do not open the sealed
pouch until you are ready to start the test.
-
Remove the test strip or cassette from the sealed foil pouch.
-
Be careful not to touch
the test development areas of the test devices with your fingers or
expose these areas to water or other fluids.
-
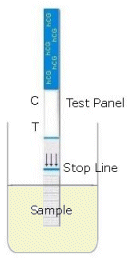
Hold the test strip
at the top in a vertical position with the arrows pointing downward.
Lower the test strip into the urine specimen. Do not immerse the test
strip beyond the "Stop Line". See illustration
at right.
-
Leave the test strip immersed for a minimum of 5 seconds. For the AimStep test card, using
the pipette provided, drop 4-5 sample drops on
the circle target area. If visible migration does not
begin within one minute, add 2-3 additional drops
-
Remove the test strip from the sample and place on a non-absorbent flat
surface.
-
Read
results for both test
formats at 3-5
minutes. Do not attempt to interpret
test results after 5 minutes.
|
|
TEST
INTERPRETATION
|
The
test is negative if only one band appears in the Control
(C) Zone. The test is positive if two colored bands appear
in the test panel (see the animated graphic to the right). One
colored band will appear in the Test (T) Zone and one in the Control
(C) Zone. Any colored band in the Test
Zone should be considered a positive
result if the band appeared within 3-5 minutes of starting the test
procedure. The colored band in the Control Zone
may be lighter or darker in color and/or intensity than the Test
Zone band; this is not relevant to test
interpretation, only the presence of a test band is important to test
interpretation. The test is invalid if no band appears in the Control Zone
even if a colored band appears in the Test Zone. This can be caused by not immersing the test strip for at least 5
seconds, immersing it beyond the "Stop Line",
or with the cassette format, not adding enough sample to the sample well.
Exposure to the test development area of the test devices to water or
other fluids, or touching these areas can contaminate and/or prevent the
proper operation of the test. |

|
QUALITY CONTROL
Each test includes a built in quality and procedural control. Correct procedural technique and test strip performance is confirmed when a colored band appears at the Control Zone of the membrane.
LIMITATIONS OF THE TESTS
-
False negative results may occur when levels of hCG are below the sensitivity level of the test. When pregnancy is still suspected, a first morning urine should be collected 48 hours later and tested.
-
Elevated levels of hCG may be found in trophoblastic disease, choriocarcinoma, and embryonal cell carcinoma. Islet cell tumors may also produce hCG as well as other carcinomas.
-
Elevated levels of hCG may remain several weeks following normal pregnancy conclusion, delivery by cesarean section, spontaneous or therapeutic abortion.
-
Ectopic pregnancies may produce very low levels of hCG. If this condition is suspected further testing using a quantitative assay may be desirable.
-
In rare
circumstances, abnormally high levels of system hCG, usually introduced
through fertility injections or other artificial means can result in a false
negative test directly attributed to the prozone effect. This possibility is
extremely rare however and occurs in a fraction of 1% of normal procedures.
EXPECTED
VALUES
Negative results are expected in healthy non-pregnant women and healthy men. Healthy pregnant women have hCG present. The
amount will vary greatly with gestational age and between individuals. First morning urine specimens approximate hCG levels which reach 5 to 50 mIU/ml within 1 week of gestational age. AimStick PBD/AimStep tests can detect pregnancy as early as 1 day after a missed menses.
PERFORMANCE CHARACTERISTICS
Accuracy:
A multi-center clinical evaluation was conducted comparing the results obtained
using AimStick PBD Pregnancy and another commercially available membrane test.
The study included 150 specimens and both assays found 72 negative and 78
positive results. AimStick PBD Pregnancy showed a 100% concordance with the
other commercially available membrane test.
SENSITIVITY AND SPECIFICITY
AimStick PBD Pregnancy detects hCG concentrations of 20 mIU/ml and greater. The test has been standardized to the World Health Organization Third International Standard
of mIU or standard international unit. The addition of LH (300 mIU/ml), FSH (1000 mIU/ml), and TSH (1000 µIU/ml) to negative (0 mIU/ml hCG) and positive (20 mIU/ml hCG) urine showed no cross-reactivity.
PRECAUTIONS
1. The test strip or card should remain in the sealed pouch until ready for use. Once the sealed pouch has been opened, the test is good for 90 days only.
2. All urine specimens should be considered potentially hazardous and handled in the same manner as an infectious waste agent.
3. The test strip should be discarded in a proper container after testing.
4. Any positive test should be confirmed with your healthcare provider immediately.
STORAGE AND STABILITY
The test strips should be stored at room temperature (15° to 30° C) for the duration of the shelf-life. The test strip must remain in the foil wrap or fog pouch until ready for use. Once the wrap or pouch has been opened the test strips are good for 90 days only.
INTERFERING SUBSTANCES
The following potential interfering substances were added to hCG negative and positive samples.
|
Acetaminophen |
20 mg/dl |
Caffeine |
20 mg/dl |
Acetylsalicylic Acid |
20 mg/dl |
|
Gentisic Acid |
20 mg/dl |
Ascorbic Acid |
20 mg/dl |
Glucose |
2 g/dl |
|
Atropine |
20 mg/dl |
Hemoglobin |
1 mg/dl |
|
|
None of the substances
above at the concentration shown interfered with test results.
|